
When you hear the phrase “aluminum charge,” what comes to mind? Maybe you picture the electrical charge that powers your favorite devices, or perhaps you think of chemistry class and the periodic table. Sounds complex? Let’s break it down. The term "aluminum charge" actually carries a dual meaning, bridging the worlds of fundamental science and everyday technology.
First, in chemistry, “aluminum charge” refers to the electrical charge that an aluminum atom carries when it becomes an ion. This is known as the ionic charge. Aluminum, with its symbol Al and atomic number 13, is famous for forming a stable ion with a +3 charge. This property shapes how aluminum interacts with other elements, determining the compounds it forms and its role in countless materials we use every day. If you’ve ever wondered why aluminum is so widely used in construction, transportation, and packaging, the answer starts with its unique charge in chemistry (source).
Second, in today’s world of electronics, “aluminum charge” also hints at the metal’s critical role in charging devices. Aluminum is a top choice for manufacturing the bodies of power banks, wireless chargers, and even the casings for laptops and smartphones. Its excellent electrical conductivity and lightweight durability make it a favorite for both engineers and everyday users.
Understanding aluminum charge in chemistry helps explain:
Meanwhile, recognizing aluminum’s role in charging devices sheds light on:
We’ll take you on a journey from the atomic structure of aluminum and the science behind its +3 charge, through to how this fundamental property translates into real-world applications—from industrial materials to the latest electronic gadgets. Along the way, you’ll see why the "aluminum charge" is at the heart of both chemistry and technology, making aluminum one of the most versatile and valuable elements in our world.
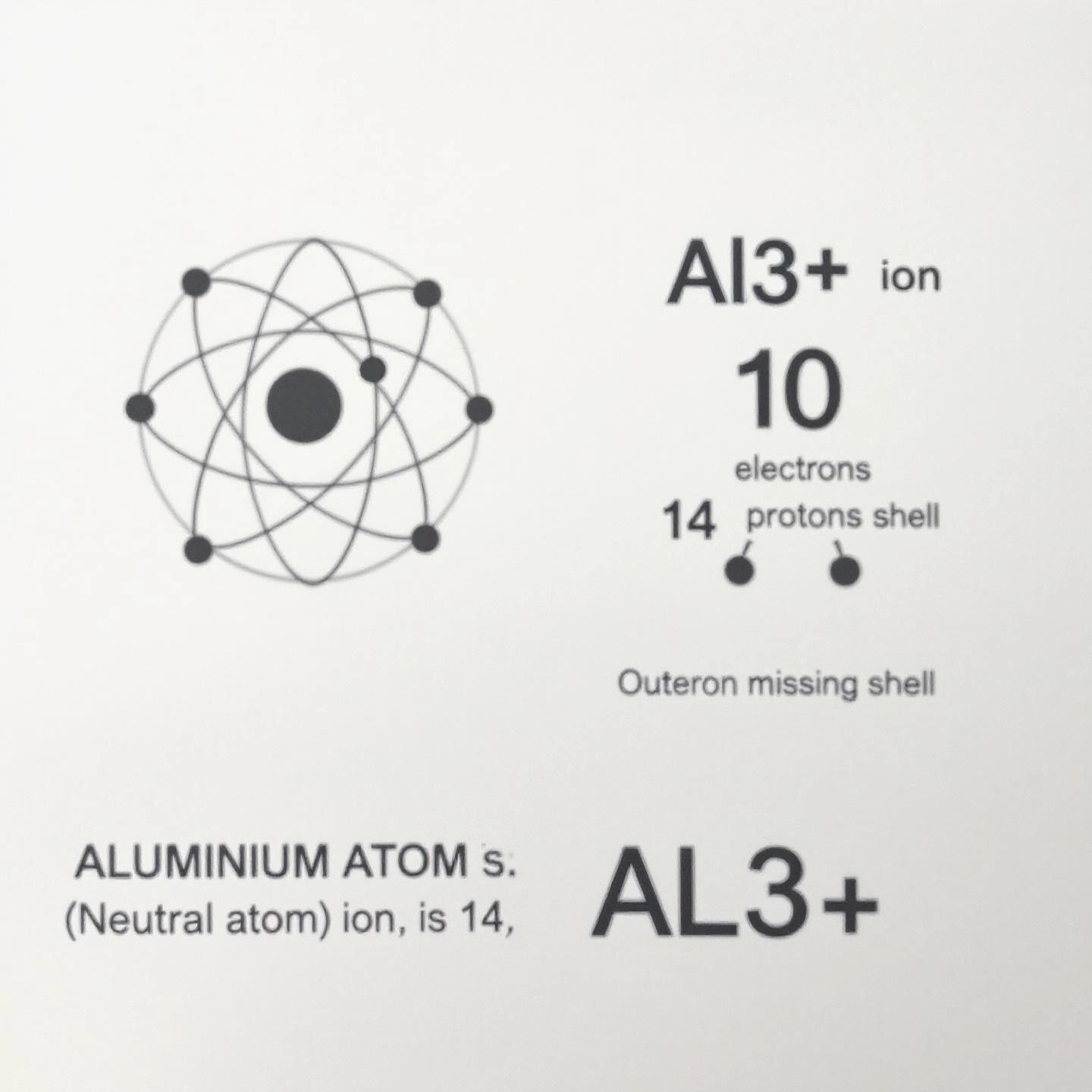
Ever wondered, "What is the charge of aluminum?" or "How does an aluminum ion differ from an aluminum atom?" These are some of the most common questions students and curious minds ask when diving into chemistry. Let’s break down the answers in simple, practical terms so you’ll never have to wonder again.
Imagine holding a pure piece of aluminum foil. At the atomic level, each aluminum atom is perfectly balanced. Here’s why:
Because the number of positive protons equals the number of negative electrons, the overall charge of a neutral aluminum atom is zero. In other words, it’s electrically neutral—neither positive nor negative.
But what happens when aluminum reacts with other elements? That’s where things get interesting. In chemical reactions, aluminum tends to lose electrons. When this occurs, the atom transforms into an ion—specifically, a cation (a positively charged ion).
This tendency to form a +3 ion is a defining feature of aluminum’s chemistry and explains why the charge of aluminum ion is always listed as +3 in textbooks and reference tables.
Still a bit fuzzy on the distinction? Here’s a quick comparison:
| Property | Aluminum Atom (Al) | Aluminum Ion (Al3+) |
|---|---|---|
| Protons | 13 | 13 |
| Electrons | 13 | 10 |
| Net Charge | 0 (neutral) | +3 (cation) |
So, when someone asks, “What charge does aluminum have?” the answer depends on whether you’re talking about the pure atom (which is neutral) or the ion (which has a +3 charge). The aluminum ion charge is always +3 in chemical compounds, which is why you’ll find Al3+ in formulas like aluminum oxide or aluminum chloride (reference).
Understanding this fundamental difference is key to grasping aluminum’s reactivity and its role in forming stable compounds. In the next section, we’ll explore why aluminum prefers to lose three electrons and how this shapes its chemical identity, setting the stage for its remarkable versatility in industry and technology.
If you’ve ever wondered, “Why does aluminum always end up with a +3 charge in compounds?” you’re not alone. The answer lies in the way atoms seek stability, and it all comes down to electrons and energy. Let’s walk through the process step by step, using simple language and relatable examples to make sense of aluminum ion formation.
First, let’s clarify a key concept: a cation is simply an atom or molecule that has lost one or more electrons, resulting in a net positive charge. In contrast, an anion is negatively charged due to gaining electrons. Metals like aluminum almost always form cations. But why?
So, when aluminum reacts, it prefers to lose three electrons rather than gain five (which would be required to fill its outer shell). Losing three is much easier and energetically favorable. This is why aluminum always forms a +3 cation (Al3+) in chemical reactions (CK-12 Chemistry).
Let’s break this down visually:
This process is known as aluminum cation formation. The equation looks like this:
Al → Al3+ + 3e-
Here, Al represents a neutral aluminum atom, Al3+ is the aluminum cation, and e- stands for the electrons that are lost.
You might ask, “Why doesn’t aluminum just gain electrons to fill its shell?” Imagine trying to carry five heavy grocery bags versus dropping off three you already have. It’s much easier for aluminum to lose three electrons than to gain five. This is why, in almost all its compounds, aluminum appears as Al3+.
Because of this tendency, you’ll notice that aluminum is almost always present as a cation in chemical compounds—think aluminum oxide (Al2O3), aluminum sulfate, and many others.
Understanding why and how the aluminum cation forms is essential for appreciating its reactivity, its role in industrial materials, and its importance in modern technology. Next, we’ll dive deeper into the atomic structure that makes this +3 charge possible, giving you a closer look at the electrons themselves.
Ever wondered why aluminum always seems to end up with a +3 charge? The answer lies deep within its atomic structure—specifically, in how its electrons are arranged. If you’ve ever asked yourself, “How does aluminum’s electron configuration determine its charge?” or “What’s so special about those valence electrons in aluminum?”, you’re about to find out.
Let’s start with the basics. Every element on the periodic table comes with a unique atomic number. For aluminum, that number is 13. This means a neutral aluminum atom has 13 protons (positively charged) in its nucleus and, to balance the charge, 13 electrons (negatively charged) orbiting around it (LibreTexts Chemistry of Aluminum).
But how are those electrons arranged? The aluminum electron configuration describes this in detail. Here’s how the electrons fill up the shells, starting from the innermost layer:
Written out, the electron configuration for aluminum is:
Or, using shorthand notation based on the noble gas neon:
Now, let’s focus on those valence electrons in aluminum. Valence electrons are the electrons in the outermost shell of an atom. For aluminum, that’s the third shell, which contains:
So, aluminum has three valence electrons. These are the electrons that participate in chemical reactions and determine how aluminum interacts with other elements.
Why does aluminum lose exactly three electrons? The answer is stability. Atoms are most stable when their outermost shell is full—this is the “octet rule” in action. For aluminum, losing its three valence electrons means its outer shell now mirrors the full shell of the noble gas neon, making it much more stable.
This is why aluminum forms the Al3+ ion in almost all of its compounds. The process can be summed up in a simple equation:
Al → Al3+ + 3e-
Here, Al is the neutral atom, Al3+ is the ion, and e- represents the electrons that are lost.
| Shell | No. of Electrons | Configuration |
|---|---|---|
| 1st (K) | 2 | 1s2 |
| 2nd (L) | 8 | 2s2 2p6 |
| 3rd (M) | 3 | 3s2 3p1 |
When all three valence electrons are lost, the third shell is emptied, and the second shell becomes the new outer shell—fully filled and stable.
Understanding the aluminum electron configuration and the behavior of its valence electrons helps explain why aluminum is so reactive and why it forms the compounds that it does. This knowledge is the foundation for understanding aluminum’s chemical properties—including its resistance to corrosion and its widespread use in technology and industry.
Next, we’ll see how this +3 ionic charge shapes aluminum’s chemical reactions and the formation of compounds like aluminum oxide, revealing why this metal is both durable and incredibly useful across so many fields.

When you hear that aluminum always forms a +3 ion, you might wonder—why does this matter in the real world? The answer lies in how the aluminum oxide charge and aluminum’s unique chemistry come together to create materials that are both reactive and incredibly durable. Let’s explore how the +3 charge shapes aluminum’s chemical properties and its everyday applications.
Aluminum is famous for its reactivity, but what drives this behavior? It all comes down to how aluminum atoms lose three electrons to form Al3+ ions. This strong positive charge makes aluminum highly attractive to negatively charged ions (anions) in its environment. In fact, as soon as bare aluminum is exposed to air, it reacts almost instantly with oxygen molecules.
The result? The formation of aluminum oxide (Al2O3), a stable compound where the +3 and –2 charges balance perfectly. The chemical equation looks like this:
2Al3+ + 3O2– → Al2O3
This reaction is not just a textbook example—it’s happening on the surface of every piece of aluminum you see!
Here’s where things get interesting. While aluminum itself is highly reactive, the aluminum oxide layer that forms on its surface is incredibly stable and unreactive. This ultra-thin coating acts as a shield, protecting the underlying metal from further reaction with air, water, or even many chemicals.
Imagine a soda can or an airplane wing—both rely on this “invisible armor” to stay strong and last for years, even in harsh environments.
Aluminum oxide isn’t just a passive protector. It’s also a versatile material with unique properties:
So, what’s the big takeaway? The +3 ionic charge is the reason aluminum is both reactive (forming bonds with other elements) and resilient (protected by its oxide layer). This duality makes aluminum a go-to material in industries where both strength and chemical resistance are essential.
Next, we’ll shift from chemistry to physics, exploring how the movement of electrons in metallic aluminum gives it another superpower: exceptional electrical conductivity. This property is at the heart of why aluminum is so valuable in everything from power lines to smartphone chargers.
Have you ever wondered why aluminum is such a popular choice for electrical wiring, power lines, and advanced tech components? The answer lies in the unique way aluminum’s electrons behave when it’s in its metallic form. Let’s break down the science behind aluminum electrical conductivity—and see why this property powers everything from your home to high-speed trains.
Up to now, we’ve focused on aluminum’s +3 ionic charge in chemical reactions. But what happens when aluminum atoms are packed together in solid metal? Here, the story shifts from ions to a remarkable phenomenon known as the "sea of delocalized electrons".
This electron "sea" is the secret to aluminum’s impressive ability to conduct electricity. When a voltage is applied, these delocalized electrons move rapidly, carrying electrical current from one end of the metal to the other (ScienceDirect).
Imagine trying to run water through a pipe—if the pipe is wide and unobstructed, the water flows easily. In a similar way, aluminum’s structure allows electrons to flow with minimal resistance. Here’s what sets it apart:
These benefits explain why aluminum for electrical wiring is now the standard in power grids, building construction, and even aerospace applications.
Aluminum’s electrical properties aren’t just theoretical—they’re at the heart of modern infrastructure and technology. You’ll notice aluminum in:
When it comes to demanding industries—like rail transit, renewable energy, or high-performance electronics—precision and quality in aluminum profiles are crucial. This is where manufacturers like Shengxin Aluminum make a difference. By combining advanced extrusion technology, strict quality control, and deep industry expertise, Shengxin delivers aluminum profiles that meet the highest standards of electrical and mechanical performance.
Whether you’re designing the next generation of electric vehicles or upgrading a city’s power grid, choosing the right aluminum profiles ensures both efficiency and reliability. As we move forward, aluminum’s unique electrical properties will continue to shape the future of technology—especially in the rapidly evolving world of charging accessories and consumer electronics.
Next, let’s see how these same properties make aluminum the preferred material for everything from wireless chargers to high-end tech gadgets, enhancing both user experience and device safety.

Ever picked up a sleek wireless charger or a lightweight power bank and wondered, "Why does it feel so premium?" Chances are, aluminum is a big part of the answer. From smartphone docks to portable battery packs, aluminum charging accessories have become the gold standard in consumer electronics—and for good reason. Let’s break down exactly why this versatile metal is the go-to choice for today’s charging solutions.
Imagine you’re designing the perfect charger. What would you want? Something that stays cool, is easy to carry, lasts a long time, and looks great on your desk. Aluminum delivers on all these fronts:
When you use an aluminum charging accessory, you’ll notice a few key benefits right away:
Consider the latest wireless charging pads. These often feature aluminum enclosures for both structural support and efficient heat dissipation. Likewise, power banks with aluminum shells are not only lighter but also more robust than their plastic counterparts. Even high-end laptop docks and multi-device charging stations use aluminum to manage heat and maintain a premium feel.
Choosing aluminum for charging accessories isn’t just about looks—it’s a smart decision for performance and safety. Aluminum’s combination of lightweight strength, heat management, and durability ensures that your devices charge efficiently and safely, day after day. As wireless and fast-charging technologies continue to evolve, expect aluminum to remain at the forefront of innovative, user-friendly designs.
Next, we’ll see how the same qualities that make aluminum a star in charging accessories also drive its adoption in high-end wearables—think fitness trackers and smartwatches—where comfort, strength, and style are all essential.
When you’re shopping for a premium aluminum charger or a unique aluminum wireless charger, it’s easy to be dazzled by sleek designs and shiny finishes. But what truly separates a top-tier charger from a basic one? Let’s dig beneath the surface to uncover what makes premium aluminum wireless chargers stand out—and why material quality, manufacturing precision, and thoughtful engineering matter more than ever.
Imagine you’ve just unboxed a new wireless charger. You might notice how cool and solid it feels in your hand, or how perfectly your phone snaps into place. These details aren’t just about aesthetics—they’re the result of:
In the world of wireless charging, these factors aren’t just “nice to have”—they directly impact safety, device compatibility, and long-term reliability.
To see the difference at a glance, check out the table below:
| Feature | Basic Aluminum Charger | Premium Aluminum Charger |
|---|---|---|
| Material Quality | Standard-grade aluminum, minimal finishing | High-grade alloy, CNC-machined, advanced surface treatment |
| Design Precision | Simple cuts, less attention to tolerances | Precision-milled, seamless fit, ergonomic details |
| Heat Dissipation | Average; may warm up during fast charging | Efficient; stays cool under heavy use |
| Finish & Aesthetics | Basic matte or brushed finish | Premium anodized, powder-coated, scratch-resistant |
| Certification & Safety | May lack full Qi certification | Qi-certified, meets global safety standards |
| Device Compatibility | Limited; may not support all cases or brands | Wide compatibility, supports fast charging for major brands |
| Longevity | Prone to wear and tear | Engineered for durability and daily use |
When you invest in a premium charger, you’re not just paying for looks. You’re getting:
What makes these high standards possible? It all comes down to advanced manufacturing capabilities. Leading producers like Shengxin Aluminum combine extensive expertise in alloy selection, extrusion, CNC machining, and finishing to deliver aluminum profiles that meet the demands of today’s tech industry. Whether it’s the ultra-thin shell of a wireless charger or the precision-milled components inside, Shengxin’s commitment to quality ensures that premium chargers aren’t just stylish—they’re engineered for performance and longevity.
As you explore the next wave of smart devices—like wearables and fitness trackers—you’ll see these same principles at work, making aluminum the material of choice for products that demand comfort, durability, and a touch of elegance.
Ever wondered why your smartwatch or fitness tracker feels so light and comfortable, yet tough enough to withstand daily life? The answer often lies in the clever use of aluminum. Today, aluminum in wearables is more than a trend—it's a key factor in making modern devices both practical and stylish. Let’s dive into why aluminum is the go-to material for high-end wearables, using real-world examples like the Fitbit Charge 6 aluminum edition to illustrate the point.
Imagine wearing a fitness tracker all day, every day. You want something that doesn’t weigh you down, looks great with every outfit, and can handle the bumps and scrapes of an active lifestyle. Aluminum delivers on all these fronts thanks to several standout properties:
Take the Fitbit Charge 6 aluminum as a prime example. This popular fitness tracker features a casing made from a combination of glass, resin, and aluminum. Here’s how aluminum enhances the user experience:
Users of the Fitbit Charge 6 often praise its comfort and durability, noting that the aluminum construction strikes the perfect balance between being feather-light and reliably tough. The device’s sleek, modern appearance is also a direct result of aluminum’s ability to be precisely machined and finished.
Aluminum’s impact goes beyond just one tracker. Across the wearable tech industry, you’ll find aluminum used in:
By combining lightweight comfort, robust protection, and customizable aesthetics, aluminum ensures that wearables not only look good but also stand up to the demands of daily life. As technology continues to advance, expect aluminum’s role in wearables to grow—pushing the limits of what’s possible in both function and fashion.
In the final section, we’ll wrap up our journey by summarizing how aluminum’s unique charge and atomic structure have paved the way for its enduring success in both industry and consumer technology.
When you look back at the journey of aluminum—from its atomic makeup to its starring role in cutting-edge technology—it’s clear why this metal is so prized across industries. Let’s quickly recap the core insights from our exploration of aluminum charge and consider what lies ahead for this versatile element.
What sets aluminum apart isn’t just its abundance or affordability—it’s the way its atomic and electronic structure translate into practical advantages. Whether you’re building skyscrapers, designing electric vehicles, or crafting the next generation of fitness trackers, aluminum’s properties provide a foundation for innovation and reliability. Its ability to be alloyed, machined, and finished with precision ensures that it can be tailored for virtually any application, from aerospace to personal electronics (Wikipedia: Aluminium).
The story of aluminum charge is far from over. As industries seek materials that balance performance, sustainability, and cost-effectiveness, aluminum will continue to lead the way. If you’re looking to source high-quality, precision-engineered aluminum profiles for your next project, partnering with experienced manufacturers like Shengxin Aluminum ensures you benefit from the latest advancements and proven reliability.
Imagine the possibilities: smarter cities, cleaner energy, lighter vehicles, and more resilient consumer tech—all powered by the unique charge and structure of aluminum. By understanding the science behind this remarkable metal, you’re better equipped to harness its potential in both today’s innovations and tomorrow’s breakthroughs.
Yes, aluminum forms a +3 charge in nearly all its compounds. This occurs because aluminum loses three electrons to achieve a stable electron configuration, resulting in an Al³⁺ ion. This +3 charge is central to aluminum's chemical behavior and its widespread use in various industries.
Aluminum has three valence electrons in its outer shell. Losing these electrons is energetically favorable, allowing aluminum to reach the stable electron arrangement of a noble gas. Gaining five electrons would require much more energy, so aluminum consistently forms a +3 cation.
The +3 charge enables aluminum to form a stable oxide layer (Al₂O₃) when exposed to air. This thin, protective coating prevents further oxidation and corrosion, making aluminum durable for construction, transportation, and electronic casings.
Aluminum’s structure allows delocalized electrons to flow easily, providing excellent electrical conductivity. Combined with its light weight, heat dissipation, and corrosion resistance, aluminum is ideal for power lines, wiring, and premium charging devices.
Premium aluminum wireless chargers use high-grade alloys, precision CNC machining, and advanced surface treatments for better heat management, durability, and aesthetics. These qualities ensure safety, fast charging, and a longer product lifespan.
 online service
online service 0086 136 3563 2360
0086 136 3563 2360 sales@sxalu.com
sales@sxalu.com +86 136 3563 2360
+86 136 3563 2360