
Ever wondered how your tap water stays crystal clear or why your favorite garden blooms so vibrantly? The answer often involves aluminum sulfate—a chemical quietly shaping both our daily routines and major industries. But what exactly is this compound, and why does it matter so much?
In simple terms, aluminum sulfate is a white, odorless crystalline salt made from aluminum, sulfur, and oxygen. You might encounter it as:
Its versatility makes it an essential ingredient in a surprising range of products and processes. For example, it helps:
Sounds complex? Don’t worry—this blog post will break down everything you need to know about aluminum sulfate. We’ll explore its chemical formula, properties, and how its unique structure enables such diverse applications. You’ll also learn practical tips for using it in gardening, water treatment, and pool maintenance. Plus, we’ll compare common variants like sodium and potassium aluminum sulfate and offer guidance on how to purchase the right product for your needs.
To dive deeper into the science and everyday impact of aluminum sulfate, check out this comprehensive blog post from Shengxin Aluminum.
Ready to unlock the full story behind this unsung chemical hero? Let’s get started by understanding what aluminum sulfate really is and why it’s so vital in both homes and industries.
When you hear the term "aluminum sulfate," you might picture a complicated lab chemical. But what is aluminum sulfate, really? Imagine opening a bag of fine, white crystals or seeing a clear solution poured into a garden sprayer—both are common ways this compound shows up in daily life.
At its core, aluminum sulfate is a salt created from aluminum, sulfur, and oxygen. It’s widely recognized for its clean, almost invisible presence in both homes and industries. You’ll notice it most often as a solid or in liquid form, depending on how it’s used. Let’s break down its essential characteristics so you know what to look for:
Aluminum sulfate’s solid form is often used in tablets or granules for water treatment and gardening, while the liquid version is ideal for quick mixing or dosing. Because it’s odorless and colorless when dissolved, it blends seamlessly into most solutions without altering their appearance or scent (source).
Now that you know what aluminum sulfate looks and feels like, let’s take a closer look at its chemical makeup and why that matters for its many uses.
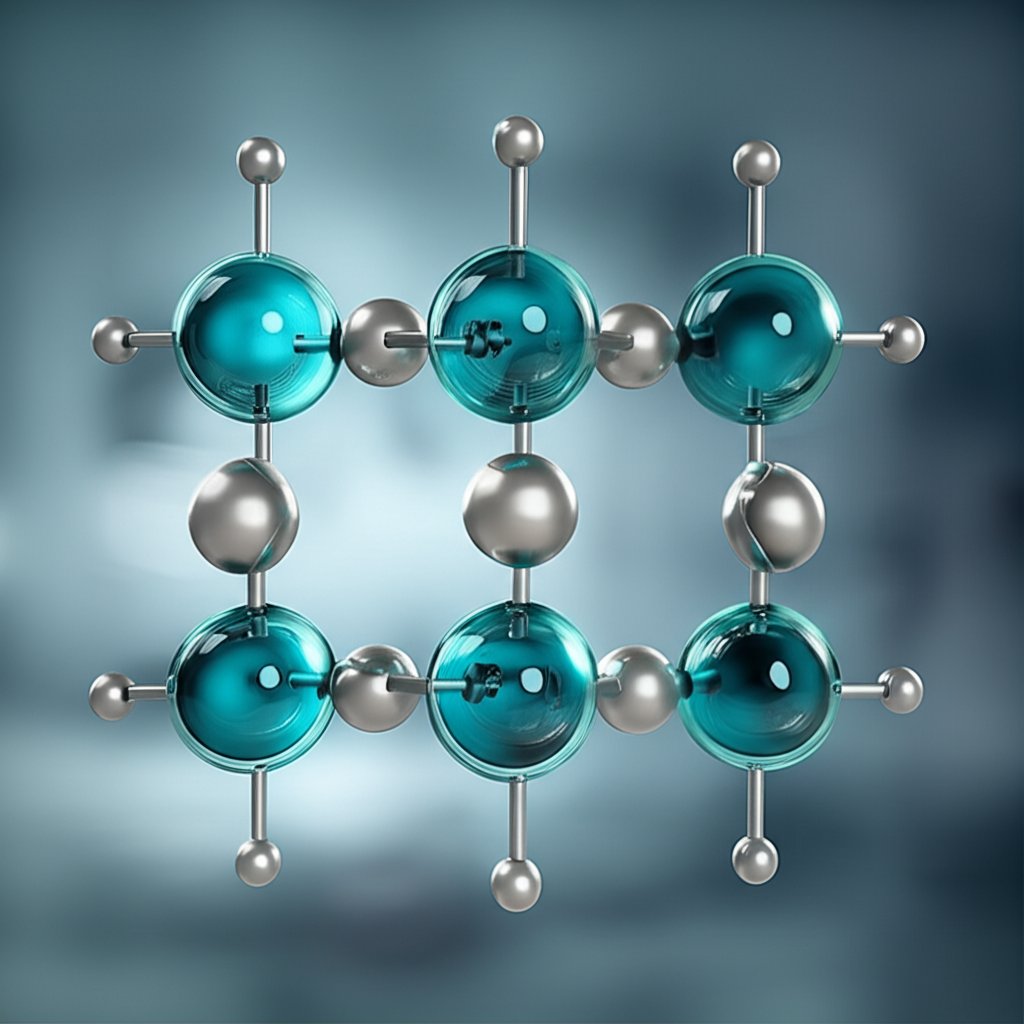
Ever wondered what gives aluminum sulfate its remarkable versatility? The answer starts with its chemical makeup. Understanding the aluminum sulfate formula not only reveals how this compound works, but also helps you see why it's so widely used across industries.
The aluminum sulfate chemical formula is Al2(SO4)3. At first glance, that might look intimidating, but let’s break it down:
So, the formula of aluminum sulfate tells us that every molecule contains:
This composition can also be written as Al2S3O12, but the standard way is Al2(SO4)3 because it highlights the sulfate groups (source).
Parentheses in the aluminum sulfate compound formula indicate that the group inside—here, the sulfate ion—appears multiple times. The subscript outside the parentheses (the 3) means you have three sulfate ions bonded to the aluminum atoms. This notation ensures the chemical structure is clear and balanced.
Now, is aluminum sulfate ionic or covalent? It’s ionic. Here’s why:
This precise balancing of charges is why the chemical formula for aluminum sulfate is always written as Al2(SO4)3 (source).
In summary, the aluminum sulfate formula—with its specific arrangement of atoms and ions—explains why this compound dissolves easily in water and interacts so effectively in everything from water treatment to gardening. Next, we’ll explore how these chemical building blocks influence its properties and reactions in real-world applications.
Ever wondered what makes aluminum sulfate such a versatile player in water treatment, gardening, and industry? The answer lies in its chemical properties—and how it reacts when mixed with water or other substances. Let’s break down the essentials in a way that’s easy to understand, even if chemistry isn’t your thing.
First, let’s look at the numbers and traits that define this compound:
Imagine you’re treating a cloudy swimming pool or purifying tap water. When you add aluminum sulfate to water, it doesn’t just dissolve—it reacts. This reaction is called hydrolysis. Here’s what happens:
This hydrolysis reaction is the reason aluminum sulfate is so effective in flocculating impurities during water treatment and in adjusting soil pH for acid-loving plants.
Aluminum sulfate’s reactivity doesn’t stop at hydrolysis. It also participates in several other chemical reactions, including:
| Property | Detail |
|---|---|
| Molar Mass | 342 g/mol (anhydrous) |
| Solubility | Highly soluble in water |
| pH of Solution | Acidic (due to hydrolysis) |
| Key Reactions | Hydrolysis, single-replacement, precipitation |
Understanding these chemical properties helps explain why aluminum sulfate is so widely used, from clarifying drinking water to supporting healthy plant growth. Next, let’s see how these traits make it especially valuable in the garden—especially for plants like hydrangeas that thrive in acidic soil.

Ever struggled to get your hydrangeas to turn that perfect blue, or wondered why some garden plants seem to thrive while others lag behind? The answer could be right under your feet—literally. Aluminum sulfate for hydrangeas and other acid-loving plants is a gardener’s secret weapon for creating vibrant blooms and lush, healthy growth.
Soil pH plays a crucial role in plant health. Many popular garden plants—like hydrangeas, azaleas, rhododendrons, blueberries, and strawberries—need acidic soil to absorb nutrients efficiently. In areas with naturally alkaline or neutral soil, these plants can struggle. That’s where aluminum sulfate for soil comes in. It quickly lowers pH, making nutrients like iron and phosphorus more available and helping your plants flourish (source).
Ever noticed how some hydrangeas are pink while others are blue? The secret lies in the soil’s pH and the presence of aluminum. For hydrangea flowers to turn blue, two things are needed: a low soil pH (acidic conditions) and available aluminum. When you add aluminum sulfate for hydrangeas, you’re providing both. Here’s how it works:
Remember, soil near concrete foundations may be more alkaline, making it harder to achieve blue blooms. Regular testing and adjustment are key.
Ready to try aluminum sulfate fertilizer in your garden? Here’s a quick checklist to get the best results:
By following these guidelines, you’ll unlock the full benefits of aluminum sulfate for plants—from spectacular hydrangea blooms to robust acid-loving shrubs and healthier soils. Next, let’s see how this versatile compound works wonders in swimming pools, helping you achieve crystal-clear water with ease.

Have you ever looked at your pool and wondered, “Why is the water still cloudy even after running the filter and adding chemicals?” If so, you’re not alone. Tiny particles—often too small for standard filters—can leave water looking dull or murky. That’s where aluminum sulfate for pools comes in, offering a time-tested solution for achieving sparkling clarity.
Imagine aluminum sulfate (often called "alum") as a magnet for microscopic debris. When added to pool water, this compound acts as a flocculant: it attracts and binds together fine particles suspended in the water, forming larger clumps (floc) that are heavy enough to settle on the pool floor. This process, known as “floccing,” is especially useful for clearing up pools after heavy storms, algae blooms, or periods of neglect (source).
Ready to restore your pool’s clarity? Here’s a practical, easy-to-follow process:
By following these steps, you’ll notice a dramatic improvement in water clarity—often overnight. The next time your pool looks less than inviting, remember that a simple alum treatment can bring back that crystal-clear sparkle. Up next, let’s see how this same flocculating power is harnessed on a larger scale in municipal and industrial water treatment systems.
When you turn on your faucet and see clear water flowing, have you ever wondered what keeps it so pure and safe? Or how cities manage the challenge of treating millions of gallons of wastewater every day? The answer often starts with a single, powerful compound: aluminum sulfate. Its use in water treatment is so widespread that over 60% of the aluminum sulfate produced in the United States is dedicated to municipal and industrial water and wastewater treatment (source).
Imagine a glass of muddy water. No matter how long you wait, the tiny particles remain suspended, making the water cloudy and unappealing. This is where aluminum sulfate steps in as a coagulant. When added to water, it reacts to form sticky particles called flocs, which trap impurities and make them heavy enough to settle out or be filtered away. This simple yet effective process is the backbone of modern water purification.
In both municipal and industrial settings, aluminum sulfate is a workhorse for tackling a wide range of water quality challenges. Here’s how it helps:
Municipalities and industries continue to rely on aluminum sulfate water treatment not just for its effectiveness, but also for its safety record and adaptability to different water conditions. Whether it’s treating river water for city distribution or purifying process water in manufacturing, its versatility is unmatched.
Of course, the success of any water treatment system depends not only on the chemicals used but also on the quality of the infrastructure itself. Imagine a treatment plant’s network of tanks, pipes, and filtration units—these require durable, corrosion-resistant materials to ensure long-term performance. That’s why high-quality industrial aluminum profiles, such as those provided by Shengxin Aluminum, are vital for constructing and maintaining water treatment facilities. Their strength, resistance to corrosion, and adaptability make them ideal for supporting the demanding environment of municipal and industrial water systems.
By understanding both the science and the supporting infrastructure behind water purification, you’ll appreciate how aluminum sulfate keeps communities safe and
 online service
online service 0086 136 3563 2360
0086 136 3563 2360 sales@sxalu.com
sales@sxalu.com +86 136 3563 2360
+86 136 3563 2360